MEDIUM
NEET
IMPORTANT
Earn 100
Assertion: bond angle is higher than bond angle in .
Reason: Oxygen is more electronegative than halogens.
(a)Both assertion and reason are true and reason is the correct explanation of assertion.
(b)Both assertion and reason are true but reason is not the correct explanation of assertion.
(c)Assertion is true but reason is false.
(d)Both assertion and reason are false.
50% studentsanswered this correctly

Important Questions on Chemical Bonding and Molecular Structure
EASY
NEET
IMPORTANT
Decreasing order of bond angle is:
MEDIUM
NEET
IMPORTANT
and are converted into monocations, and respectively. Which of the following is wrong?
MEDIUM
NEET
IMPORTANT
and are converted into mono anions, and respectively. Which of the following statements is wrong?
EASY
NEET
IMPORTANT
Which one of the following arrangements of molecules is correct on the basis of their dipole moments?
MEDIUM
NEET
IMPORTANT
In piperidine 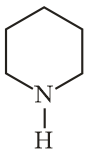 , the hybrid state assumed by is
, the hybrid state assumed by is
MEDIUM
NEET
IMPORTANT
Assertion: Bond angle of is smaller than .
Reason: Electronegativity of the central atom increases, bond angle decreases.
MEDIUM
NEET
IMPORTANT
The shapes of and respectively are
HARD
NEET
IMPORTANT
Consider the statements :
I. Bond length in is greater than in
II. Bond length in is less than in
III. has shorter bond length than
Which of the following statements are true?
