EASY
Earn 100
Assertion: The internal energy of an isothermal process does not change.
Reason: The internal energy of a system depends only on pressure of the system
(a)If both Assertion and Reason are true and the Reason is correct explanation of the Assertion.
(b)If both Assertion and Reason are true but Reason is not explanation of the Assertion.
(c)If Assertion is true but the Reason is false.
(d)If Assertion is false but Reason is true.
77.78% studentsanswered this correctly
Important Questions on Chemical thermodynamics and energetics
EASY
The quantity of heat (in ) required to raise the temperature of of ethanol from to the boiling point and then change the liquid to vapor at that temperature is closest to [Given, boiling point of ethanol . Specific heat capacity of liquid ethanol . Latent heat of vaporisation of ethanol ]
MEDIUM
EASY
EASY
MEDIUM
EASY
EASY
(a) Internal energy and enthalpy each depends on temperature.
(b) Compressibility factor is not equal to 1
(c)
(d) for any process
MEDIUM
MEDIUM
MEDIUM
EASY
MEDIUM
[Heat of fusion of ice ; Specific heat of water ]
MEDIUM
HARD
MEDIUM
of is mixed with of The molar heat of neutralization of this reaction is The increase in temperature in of the system on mixing is The value of is (Nearest integer)
[Given: Specific heat of water
Density of water]
(Assume no volume change on mixing)
MEDIUM
MEDIUM
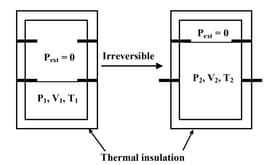
EASY
MEDIUM
EASY

