Assertion (A):- is readily soluble in water
Reason (R):- The greater hydration enthalpy of ions overcome its lattice enthalpy.
Important Questions on The s - Block Elements (Alkali and Alkaline Earth Metals)
Identify and respectively in the following reactions
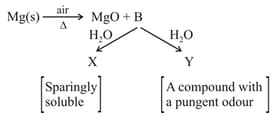
Given below are two statements:
Statement I : Both and undergo dehydration on heating.
Statement II : is amphoteric, whereas the oxides of other elements in the same group are acidic.
In the light of the above statements, choose the correct answer from the options given below:
Given below are two statements:
Statement I : None of the alkaline earth metal hydroxides dissolve in alkali.
Srtatement II : Solubility of alkaline earth metal hydroxides in water increases down the group.
In the light of the above statements, choose the most appropriate answer from the options given below:
Match List with List :
| List | List | ||
| Acidic | |||
| Basic | |||
| Amphoteric | |||
Choose the most appropriate answer from the options given below
Number of amphoteric compounds among the following is
(A)
(B)
(C)
(D)
An orange solid on heating gives a green residue a colourless gas and water vapours. The dry gas upon pasing over heated gave a white solid which upon subsequent reaction with water gave a gas that gave dense white fumes with .
Identify
Assertion (A): Barium carbonate is insoluble in water and is highly stable.
Reason (R): The thermal stability of the carbonates increases with increasing cationic size.
Choose the most appropriate answer from the options given below :

