EASY
Earn 100
Assertion (A) : Helium & Beryllium have similar outer electronic configuration
Reason (R) : Both are chemically inert
(a)
(b)
(c)
(d)
50% studentsanswered this correctly
Important Questions on The p-Block Elements
MEDIUM
| Column-I | Column-II | ||
| pyramidal | |||
| square planar | |||
| distorted octahedral | |||
| square pyramidal | |||
EASY
MEDIUM
EASY
EASY
MEDIUM
HARD
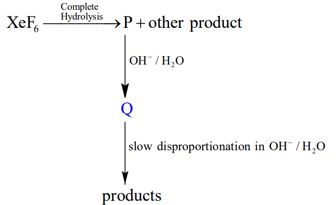
Under ambient conditions, the total number of gases released as products in the final step of the reaction scheme shown below is
EASY
MEDIUM
| Column I | Column II | ||
| (a) | (i) | Distorted octahedral | |
| (b) | (ii) | Square planar | |
| (c) | (iii) | Pyramidal | |
| (d) | (iv) | Square pyramidal |
EASY
EASY
Write the chemical equation of the following reaction for mixing and .
EASY
EASY
MEDIUM
MEDIUM
Among the following molecules,
(i)
(ii)
(iii)
those having same number of lone pairs on are:
EASY
MEDIUM
EASY
MEDIUM
Match the following?
| Reaction | Oxidation state of in product | ||
| (a) | (i) | ||
| (b) | (ii) | ||
| (c) | (iii) | ||
EASY

