HARD
Earn 100
Assertion. is planar while is pyramidal.
Reason. in has and in has hybridization.
60.47% studentsanswered this correctly
Important Questions on Chemical Bonding and Molecular Structure
EASY
MEDIUM
EASY
(i)
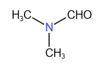 (ii)
(ii) 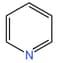
(iii) (iv)
EASY
EASY
EASY
EASY
MEDIUM
MEDIUM
EASY
MEDIUM
EASY
Draw the shape of the following molecule:
EASY
| Column I | Column II |
| a. | (i) T-shape |
| b. | (ii) Pentagonal bipyramidal |
| c. | (iii) Linear |
| d. | (iv) Square – pyramidal |
| (v) Tetrahedral |
MEDIUM
EASY
HARD
EASY
MEDIUM
EASY
EASY
Draw the shape of the following molecule:
