MEDIUM
Earn 100
Assertion. The ion exists in the solid-state and also in the liquid state but not in an aqueous solution.
Reason. The magnitude of hydrogen bonds in between molecules are weaker than that in between and molecules.
(a)If both assertion and reason are correct, and reason is the correct explanation of the assertion.
(b)If both assertion and reason are correct, but reason is not the correct explanation of the assertion.
(c)If assertion is correct, but reason is incorrect.
(d)If both assertion and reason are incorrect.
50% studentsanswered this correctly
Important Questions on States of Matter
EASY
[ is the distance between the polar molecules]
EASY
Increasing order of boiling points in the following compounds is:
EASY
MEDIUM
EASY
MEDIUM
Given:
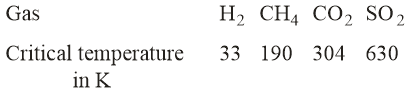
On the basis of data given above, predict which of the following gases shows the least adsorption on a definite amount of charcoal?
MEDIUM
MEDIUM

EASY
EASY
HARD
EASY
MEDIUM
MEDIUM
In which of the following solid substance dispersion forces exist?
EASY
(Latent heat of ice is and )
MEDIUM
The combination of plots which does not represent isothermal expansion of an ideal gas is




HARD
MEDIUM
Match the type of interaction in column with the distance dependence of their interaction energy in column
| A | B |
| (i) ion - ion | (a) |
| (ii) Dipole - dipole | (b) |
| (iii) London dispersion | (c) |
| (d) |
EASY
EASY

