EASY
MHT-CET
IMPORTANT
Earn 100
Assign oxidation number to atoms of only those elements which undergo oxidation number change in the following redox reactions, and then balance the equations.

Important Questions on Redox Reactions
EASY
MHT-CET
IMPORTANT
EASY
MHT-CET
IMPORTANT
MEDIUM
MHT-CET
IMPORTANT
Given:
Which of the following is correct?
MEDIUM
MHT-CET
IMPORTANT
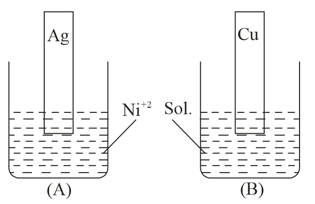
Two beakers, and each contain a nickel ion solution. A silver strip is dipped in beaker and a copper strip is dipped in beaker .
Given that,
and .
Based on the above information, which of the following is correct?
MEDIUM
MHT-CET
IMPORTANT
MEDIUM
MHT-CET
IMPORTANT
a)
b)
c)
Based on the data given above, what is the correct order of reducing power?
EASY
MHT-CET
IMPORTANT
What is the meaning of a positive sign for the half-cell potential?
EASY
MHT-CET
IMPORTANT
What is the meaning of a positive sign for cell potential?