At , a litre solution containing of and of shows a of .
Given : and
The potential for the half cell reaction is . The value of is _____ .

Important Questions on Electrochemistry
Consider the cell
Given: and
If the potential of the cell is the ratio of concentration of to is
(Nearest integer)
The for the given cell is at
When
The value of a is ___________
Given :
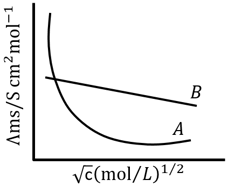
Following figure shows dependence of molar conductance of two electrolytes on concentration. is the limiting molar conductivity.
The number of Incorrect statement(s) from the following is _____
(A) for electrolyte is obtained by extrapolation
(B) For electrolyte graph is a straight line with intercept equal to
(C) At infinite dilution, the value of degree of dissociation approach zero for electrolyte .
(D) for any electrolyte or can be calculated using for individual ions.
The equilibrium constant for the reaction is at . The magnitude of standard electrode potential of if is _____ . (Nearest integer)
Given :
Consider the cell
When the potential of the cell is at , the ratio is
(Nearest integer)
Given:
The electrode potential of the following half cell at
is _____ (Nearest integer)
Given :
Which one of the following statements is correct for electrolysis of brine solution?