MEDIUM
Earn 100
At , the root mean square speed of gas (molar mass ) is equal to the most probable speed of gas at . The molar mass of the gas is _____ . (Nearest integer)
25% studentsanswered this correctly
Important Questions on Some Basic Concepts of Chemistry
EASY
MEDIUM
EASY
MEDIUM
EASY
MEDIUM
EASY
MEDIUM
EASY
HARD
If the distribution of molecular speeds of a gas is as per the figure shown below, then the ratio of the most probable, the average, and the root mean square speeds, respectively, is
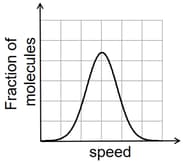
EASY
MEDIUM
EASY
MEDIUM
Represent the union of two sets by Venn diagram for each of the following.
is a prime number between and
is an odd number between and
EASY
EASY
HARD
MEDIUM
EASY
MEDIUM

