HARD
Earn 100
At a certain temperature , some hydrazine , gas is placed in a sealed flask where the following equilibrium is established
If the temperature is increased to T2, some ammonia gas also decomposes as
What would be observed as temperature is increased from T1 to T2?
(a)Some more hydrazine would decompose.
(b)If , concentration of would decrease.
(c)Partial pressure of N2 would be increased.
(d)All of these would be observed.
50% studentsanswered this correctly
Important Questions on Equilibrium
MEDIUM
The total pressure when both the solids dissociate simultaneously is:
HARD
at is given below.
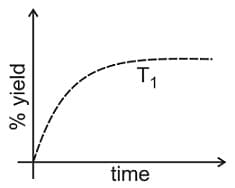
If this reaction is conducted at with , the % yield of ammonia as a function of time is represented by:
EASY
EASY
Consider the following reaction:
For each of the following cases , the direction in which the equilibrium shifts is:
(a) Temperature is decreased.
(b) Pressure is increased by adding at constant T.
MEDIUM
HARD
Assertion: The of water increases with increase in temperature.
Reason: The dissociation of water into and is an exothermic reaction.
MEDIUM
Which of the following statements is incorrect?
EASY
EASY
Using the Le Chatelier's principle, predict which one of the following will not disturb the equilibrium?
EASY
EASY
The volume of a closed reaction vessel in which the following equilibrium reaction occurs is halved.
As a result,
EASY
MEDIUM
Following lists contain reactions and their corresponding equilibrium constants at different temperatures:
| List - I (Reaction) | List - II |
If are the standard enthalpies for the reactions respectively, then:
EASY
The equilibrium shifts in forward direction:
EASY
In a reaction Le Chatelier's principle asserts that an equilibrium between and producing and can be shifted towards and by
(i) increasing the concentration of or
(ii)increasing the concentration of or
(iii) decreasing the concentration of or
MEDIUM
EASY
The equilibrium constant cannot be disturbed by
HARD
EASY
MEDIUM

