MEDIUM
Earn 100
At a fixed concentration, the molar conductance of aqueous sodium hydroxide at is found to beAt infinite dilution and its molar conductance is found to be Find the degree of ionization of sodium hydroxide at the same concentration and temperature.
(a)
(b)
(c)
(d)
50% studentsanswered this correctly
Important Questions on Electrochemistry
EASY
HARD
MEDIUM
MEDIUM
HARD
EASY
MEDIUM
HARD
A aqueous solution of has a conductance of when measured in a cell constant . The molar conductivity of this solution is _______ . (Round off to the Nearest Integer)
EASY
The variation of molar conductively with concentration of an electrolyte (X) in aqueous solution is shown in the given figure.
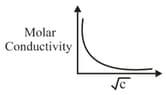
The electrolyte X is :
EASY
MEDIUM
EASY
: Conductivity always increases with decreases in the concentration of electrolyte.
: Molar conductivity always increases with decreases in the concentration of electrolyte.
The correct option among the following
MEDIUM
EASY
EASY
MEDIUM
Given below are two statements:
Statement I : The limiting molar conductivity of (strong electrolyte) is higher compared to that of $\mathrm{CH}_{3} \mathrm{COOH}$ (weak electrolyte).
Statement II : Molar conductivity decreases with decrease in concentration of electrolyte.
In the light of the above statements, choose the most appropriate answer from the options given below:
EASY
MEDIUM
MEDIUM
HARD

