HARD
Earn 100
At equilibrium, the concentrations of reactants and products become equal.
(a)True
(b)False
50% studentsanswered this correctly
Important Questions on Equilibrium
MEDIUM
EASY
At equilibrium, the mass of each of the reactants and products remains constant.
At equilibrium, the rate of forward reaction is equal to the rate of backward reaction.
MEDIUM
Using the data provided, find the value of equilibrium constant for the following reaction at and atm pressure.
MEDIUM
When and are compared at It is found that
EASY
MEDIUM
EASY
The rate of this reaction is increased by
MEDIUM
MEDIUM
The value of for the reaction
The value of for the following reaction is :
HARD
The reaction
is in equilibrium in a closed vessel at The partial pressure (in atm) of in the reaction vessel is closest to:
[Given: The change in Gibbs energies of formation at and bar for
Gas constant ]
HARD
For this equilibrium, the correct statement(s) is/are
EASY
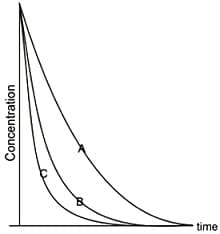
These profiles imply that the decay constants and follow the order
MEDIUM
MEDIUM
Briefly explain the following:
Dynamic nature of chemical equilibrium
HARD
HARD
HARD
MEDIUM
HARD
Classify the processes happening in the given situations as physical and chemical equilibrium.
(a) The equilibrium in a sealed soda bottle.
(b) The dissociation of in a closed container.
(c) Boiling of water in a closed beaker.
(d) and gas enclosed in a glass bulb at .
EASY

