EASY
Earn 100
At high concentration of soap in water soap behaves as a
(a)Multi molecular colloid
(b)Associated colloid
(c)Macromolecular colloid
(d)Lyophilic colloid
50% studentsanswered this correctly
Important Questions on Surface Chemistry
EASY
Match the following:
| List- | List- | ||
| Type of Colloid | Phase in medium | ||
| () | Aerosol | () | Solid in solid |
| () | Emulsion | () | Liquid in solid |
| () | Foam | () | Gas in liquid |
| () | Gel | () | Solid in gas |
| () | Liquid in liquid |
The correct answer is
EASY
EASY
EASY
EASY
EASY
EASY
EASY
Match List I with List II :
List-I List-II
Example of colloids Classification
(a) Cheese (i) dispersion of liquid in liquid
(b) Pumice stone (ii) dispersion of liquid in gas
(c) Hair cream (iii) dispersion of gas in solid
(d) Cloud (iv) dispersion of liquid in solid
Choose the most appropriate answer from the options given below
EASY
Match the following:
| (i) Foam | (a) smoke |
| (ii) Gel | (b) cell fluid |
| (iii) Aerosol | (c) jellies |
| (iv) Emulsion | (d) rubber |
| (e) froth | |
| (f) milk |
MEDIUM
MEDIUM
EASY
EASY
EASY
EASY
MEDIUM
MEDIUM
Identify the correct molecular picture showing what happens at the critical micellar concentration of an aqueous solution of a surfactant

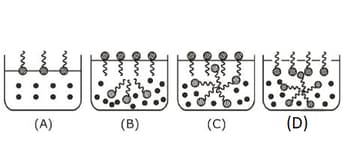
MEDIUM
(Critical micelle concentration (CMC) is marked with an arrow in the figures.)
EASY

