HARD
Earn 100
At the point of intersection of the two curves shown, the concentration of is given by………for,
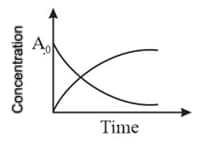

(a)
(b)
(c)
(d)
50% studentsanswered this correctly
Important Questions on Chemical Kinetics
MEDIUM
MEDIUM
HARD
MEDIUM
The given plots represent the variation of the concentration of a reactant with time for two different reactions . The respective orders of the reaction are
(i) 
(ii) 
EASY
MEDIUM
EASY
MEDIUM
EASY
EASY
MEDIUM
HARD
MEDIUM
MEDIUM
EASY
EASY
MEDIUM
EASY
is zero
EASY
EASY

