MEDIUM
Earn 100
Azoxybenzene can be obtained by the treatment of nitrobenzene with:
(a)O2
(b)H2/Pt
(c)Na3AsO3/NaOH
(d)Zn/NaOH
50% studentsanswered this correctly
Important Questions on Organic Nitrogen Compounds
HARD
The major product obtained in the following reaction is:
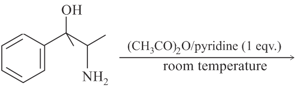
HARD
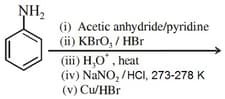
MEDIUM
What will be the major product in the following mononitration reaction?
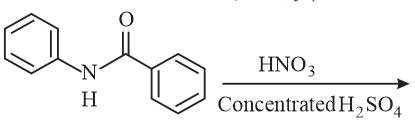
MEDIUM
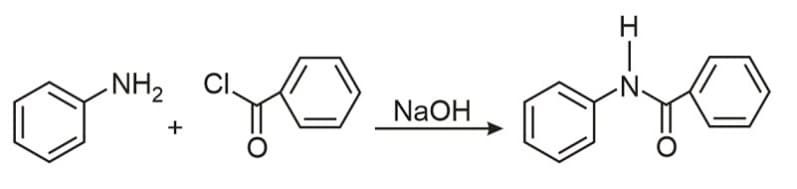
Is known by the name:
MEDIUM
HARD
Aniline reacts with mixed acid (conc. and conc. ) at . The major product(s) of the following reaction sequence is (are)
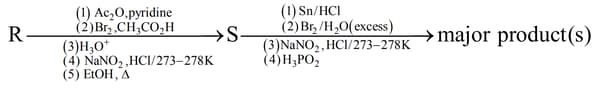
MEDIUM
The major product formed in the reaction given below will be :
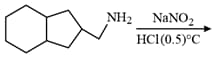
MEDIUM
EASY
HARD
EASY
HARD
Write structural formula of the compounds to .
HARD
HARD
The major product of the following reaction is:
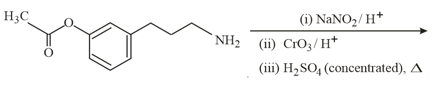
HARD
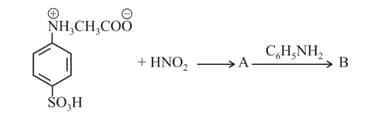
MEDIUM
EASY
HARD
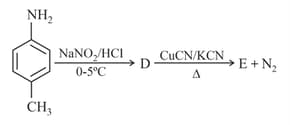
The product is
EASY
In the reaction given below, is
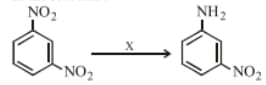
MEDIUM

