.
Choose from the following:
A:
B:
Important Questions on p-Block Elements - I
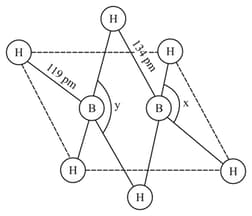
The structure of diborane is given below. Identify the bond angles In diborane, which bonds are commonly known as banana-bonds
| Column-1 Reaction |
Column-2 Main product |
||
| A | I | ||
| B | II | ||
| C | III | ||
| IV | |||
| V |
The correct match is
Identify the correct statement for from those given below.
(A) In , all bonds are equivalent.
(B) In , there are four -centre- -electron bonds.
(C) is a Lewis acid.
(D) can be synthesized from both and .
(E) is a planar molecule.
Choose the most appropriate answer from the options given below :
Given below are the statements about diborane
(a) Diborane is prepared by the oxidation of with
(b) Each boron atom is in hybridized state
(c) Diborane has one bridged centre--electron bond
(d) Diborane is a planar molecule
The option with correct statement(s) is
Which of the following reactions can be used to prepare diborane?
The geometry around boron in the product '' formed from the following reaction is
I. It is prepared by the oxidation of sodium borohydride with iodine.
II. It undergoes cleavage reactions with Lewis bases to give borane adducts.
III. It is produced on an industrial scale by the reaction of with .
IV. It is readily hydrolysed by water to give borazine.
V. It burns in oxygen and gives boron trioxide.

