MEDIUM
Earn 100
molecule is formed by hybrid orbitals?
(a)True
(b)False
50% studentsanswered this correctly
Important Questions on Chemical Bonding and Molecular Structure
EASY
EASY
| Column I | Column II |
| a. | (i) T-shape |
| b. | (ii) Pentagonal bipyramidal |
| c. | (iii) Linear |
| d. | (iv) Square – pyramidal |
| (v) Tetrahedral |
HARD
EASY
MEDIUM
EASY
MEDIUM
EASY
EASY
EASY
(i)
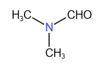 (ii)
(ii) 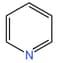
(iii) (iv)
MEDIUM
EASY
Draw the shape of the following molecule:
EASY
How many (i) hybridised carbon atoms and (ii) bonds are present in the following compound?
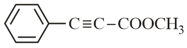
MEDIUM
MEDIUM
EASY
MEDIUM
MEDIUM
EASY
EASY
Draw the shape of the following molecule:

