HARD
11th CBSE
IMPORTANT
Earn 100
Balance the following equation in the basic medium by ion-electron method and oxidation number methods and identify the oxidizing agent and the reducing agent.

Important Questions on Redox Reactions
HARD
11th CBSE
IMPORTANT
HARD
11th CBSE
IMPORTANT
HARD
11th CBSE
IMPORTANT
HARD
11th CBSE
IMPORTANT
HARD
11th CBSE
IMPORTANT
Consider the elements :
and
Identify the element that exhibits only negative oxidation state, identify the element that exhibits only positive oxidation state, identify the element that exhibits both positive and negative oxidation states and identify the element which exhibits neither the negative nor does the positive oxidation state.
HARD
11th CBSE
IMPORTANT
HARD
11th CBSE
IMPORTANT
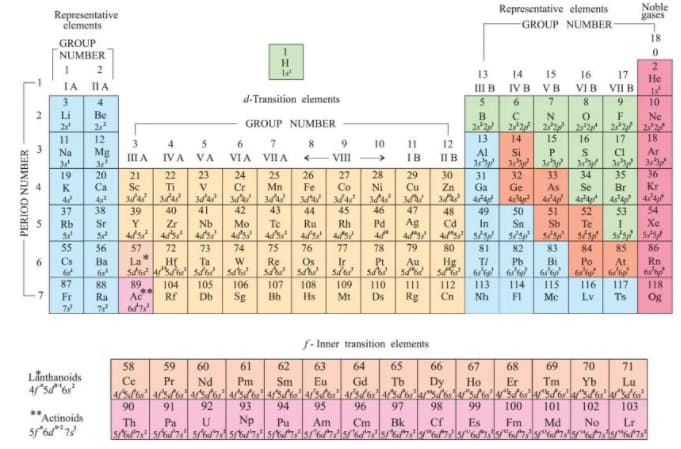
Refer to the periodic table given above, and now answer the following questions:
(a) Select the possible non-metals that can show disproportionation reaction.
(b) Select three metals that can show disproportionation reaction.
HARD
11th CBSE
IMPORTANT
