MEDIUM
Earn 100
Benzene diazonium chloride in aqueous solution decomposes as :
The reaction follows first order kinetics. If pt is the pressure of N2 at constant volume and temperature corresponding to different intervals of time t and pf that after completion of the reaction, then which of the following graphs conforms to the kinetics of the reaction ?
(a)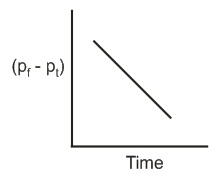

(b)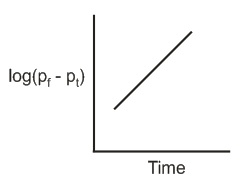

(c)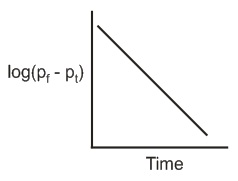

(d)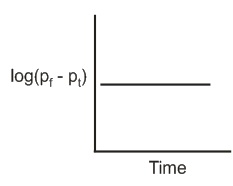

50% studentsanswered this correctly
Important Questions on Chemical Kinetics
MEDIUM
The rate constant for the first order reaction is How much time will it take to reduce the concentration of the reactant to value?
MEDIUM
For a zero - order reaction with rate constant k, the slope of the plot reactant concentration against time is
HARD
The half-life period of a first-order reaction is . The amount of substance left after one hour will be
MEDIUM
The rate of a first-order reaction is at second and at seconds after initiation of the reaction. The half-life period of the reaction is:
EASY
If 50 % of a reaction occurs in 100 second and 75 % of the reaction occurs in 200 second, the order of this reaction is:
MEDIUM
The given plots represent the variation of the concentration of a reactant with time for two different reactions . The respective orders of the reaction are
(i) 
(ii) 
EASY
A first-order reaction has a specific reaction rate How much time will it take for the reactant to reduce to ?
MEDIUM
A flask contains a mixture of compounds and . Both compounds decompose by first-order kinetics. The half-lives for and are and , respectively. If the concentrations of and are equal initially, the time required for the concentration of A to be four times that of (in s) is :
EASY
It takes for a first order reaction to go to completion. The total time required for the same reaction to reach completion will be
EASY
The rate constant for a first order reaction is If initial concentration of the reactant is , what is the half-life of the reaction?
MEDIUM
If the rate constant for a first order reaction is , the time required for the completion of of the reaction is given by
HARD
The reaction follows first order kinetics. The pressure of a vessel containing only was found to increase from 50 mm Hg to 87.5 mm Hg in 30 min. The pressure exerted by the gases after 60 min. Will be (Assume temperature remains constant) :
MEDIUM
The rate constant of a first order reaction is . How long will of this reactant reduce to ?
MEDIUM
Decomposition of follows a first order reaction. In fifty minutes the concentration of decreases from to in one such decomposition. When the concentration of reaches , the rate of formation of will be:
EASY
The half-life for a zero order reaction having initial concentration of reactant is . The rate constant (in ) for the reaction is
MEDIUM
The rate constant for a first order reaction is . The time required to reduce of the reactant to is :
EASY
Under what condition the order of the reaction,
is zero
EASY
When initial concentration of a reactant is doubled in a reaction, its half-life period is not affected. The order of the reaction is:
EASY
The rate constant of the reaction is mole per liter per second. If the initial concentration of A is 5 M, then the concentration of B after 20 minutes is:

