MEDIUM
Earn 100
Benzene diazonium chloride on treatment with in the presence of cuprous ions gives
(a)Phenol
(b)Aniline
(c)Benzene
(d)Chlorobenzene
40% studentsanswered this correctly
Important Questions on Hydrocarbons
EASY

EASY
EASY
EASY
Identify the product obtained in following reaction
EASY
EASY
MEDIUM
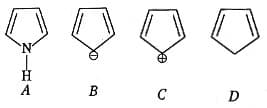
MEDIUM
Among the following, the reaction that proceeds through an electrophilic substitution, is:
EASY
MEDIUM
EASY
The major product of the following reaction is:
EASY
MEDIUM
(A is a lowest molecular weight alkyne)
EASY
MEDIUM
MEDIUM
The major product obtained in the given reaction is:
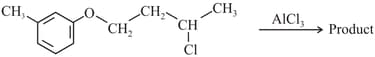
MEDIUM
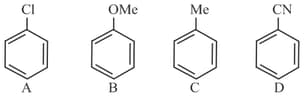
EASY
In the following reactions
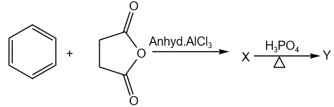
and , respectively, are:
MEDIUM
The major product of the following reaction is:
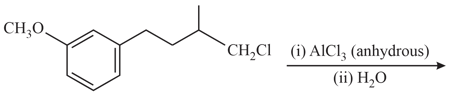
HARD
The number of hybridized carbon atom(s) present in the product is

