EASY
Earn 100
Beryllium reacts with water and form Beryllium hydroxide.
(a)True
(b)False
50% studentsanswered this correctly
Important Questions on The s-Block Elements
EASY
EASY
EASY
EASY
EASY
HARD
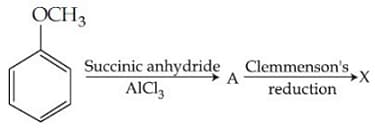
X is :
MEDIUM
EASY
EASY
MEDIUM
MEDIUM
A metal M readily forms water soluble sulphate Water insoluble hydroxide and oxide MO which becomes inert on heating. The hydroxide is soluble in NaOH. Then M is
MEDIUM
MEDIUM
MEDIUM
EASY
HARD
Beryllium and aluminium exhibit many properties which are similar. But, the two elements differ in:
MEDIUM
EASY
MEDIUM
EASY

