HARD
Earn 100
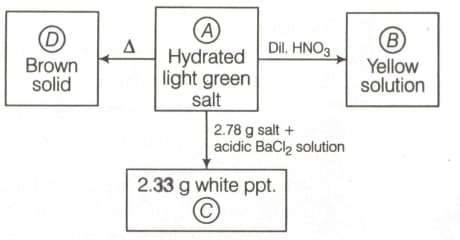
Brown ppt. (A) dissolve in HNO3 gives (B) which gives white ppt. (C) with NH4OH. (C) on reaction with HCl gives solution (D) which gives white turbidity on addition of water. What is (D) ?
(a)BiCl3
(b)Bi(OH)3
(c)BiOCl
(d)Bi(NO3)3
33.33% studentsanswered this correctly
Important Questions on Theoretical Principles of Experimental Chemistry
EASY
Which one of the following compounds is not a yellow colored compound ?
MEDIUM
Copper sulphate solution reacts with to give
EASY
Match List - I with List - II :
| List - I (Metal ion) | List - II (Group in Qualitative analysis) | ||
| (a) | (i) | Group - III | |
| (b) | (ii) | Group - IIA | |
| (c) | (iii) | Group - IV | |
| (d) | (iv) | Group - IIB |
HARD
A solution containing a group cation gives a precipitate on passing A solution of this precipitate in dil. produces a white precipitate with solution and bluish-white precipitate with basic potassium ferrocyanide. The cation is
HARD
Acidic ferric chloride solution on treatment with excess of potassium ferrocyanide gives a Prussian blue coloured colloidal species. It is:
MEDIUM
When gas is passed into a mixture of and ion in an acidified aqueous solution, the precipitates formed are
MEDIUM
cation gives a prussian blue precipitate on addition of potassium ferrocyanide solution due to the formation of
MEDIUM
When gas is passed through a hot acidic aqueous solution containing and a precipitate is formed which consists of
MEDIUM
The pair(s) of ions where BOTH the ions are precipitated upon passing gas in presence of dilute , is (are)
EASY
The Nessler's reagent contains
MEDIUM
The incorrect statement is:
EASY
To an aqueous solution containing ions such as and was added , followed by . The total number of cations precipitated during this reaction is/are :
MEDIUM
During the qualitative analysis of salt with cation , addition of a reagent to alkaline solution of the salt gives a bright red precipitate. The reagent and the cation present respectively are :
HARD
In the following reaction sequence in aqueous solution, the species and respectively, are

HARD
The reaction of and in water produces a precipitate that dissolves upon the addition of of appropriate concentration. The dissolution of the precipitate is due to the formation of
MEDIUM
For the following Assertion and Reason, the correct option is
Assertion (A) : When (II) and sulphide ions are mixed, they react together extremely quickly to give a solid.
Reason (R) : The equilibrium constant of is high because the solubility product is low.
MEDIUM
Upon treatment with ammoniacal , the metal ion that precipitates as a sulphide is
HARD
An aqueous solution of a salt X turns blood red on treatment with and blue on treatment with , X also gives a positive chromyl chloride test. The salt X is: