By means of simple diagrams explain the formation of carbon tetrachloride.

Important Questions on Chemical Bonding
If and are elements given that is an inert gas (not helium)
| Element | Atomic no. |
What type of bonding would take place between (i) and and (ii) and .
Draw diagram to illustrate the structure of molecule of Ammonia.
The distribution of electrons in the atoms of three elements is given below:
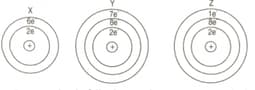
For answering the following questions you are not required to identify the atoms. You may use the letters and as symbols for the elements. In each case given below state whether the bonding is ionic or covalent and give the formula of the molecules of the compound so formed.
(a) Between two atoms of .
(b) Between the atom of and the atom of .
(c) Between the atom of and the atom of .
Complete the following statements:
(a) The bond which is formed by transfer of an electron from one atom to another atom is called _______.
(b) The atoms and become ions. Thus, ______ and _______.
(c) The formula of the compound is _____.
The following tables give information about the properties of some chemicals.
| Chemical | Electrical Conductivity | Melting Point | |||
| Solid | Liquid | Solution in water | |||
| Poor | Good | Good | |||
| Good | Good | Not Soluble | |||
| Good | Good | Not Soluble | |||
| Poor | Poor | Good | |||
| Poor | Good | Good | |||
| Poor | Poor | Not soluble | |||
| Poor | Poor | Not Soluble | |||
The properties listed are the ability of the chemical to conduct electricity as a solid, as a liquid (i.e., molten), as a solution in water. The melting point of each chemical is given in the fifth column.
Study the above table carefully and then answer the questions below. Use the letters given in the first column of the table to indicate your choice.
(a) Which of the above chemicals could be a metal?
(b) Which chemicals have a molecular structure?
(c) Which chemicals seem to change from molecular structure to ions when dissolved?
(d) Which chemicals could be definitely not be an element?
How do metals differ from non-metals in ion information.
