HARD
Earn 100
By what factor does the average velocity of a gaseous molecule increase when the absolute temperature is doubled?
(a)1.4
(b)2.0
(c)2.8
(d)4.2
50% studentsanswered this correctly
Important Questions on Atoms and Molecules
EASY
MEDIUM
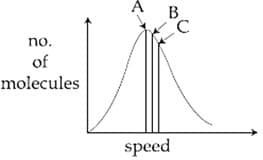
Root mean square speed most proable speed Average speed
EASY
MEDIUM
EASY
MEDIUM
HARD
If the distribution of molecular speeds of a gas is as per the figure shown below, then the ratio of the most probable, the average, and the root mean square speeds, respectively, is
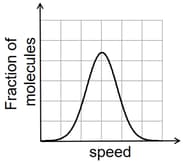
MEDIUM
MEDIUM
EASY
MEDIUM
MEDIUM
EASY
EASY
EASY
MEDIUM
EASY
EASY

