MEDIUM
Earn 100
Calculate the number of geometrical isomers for a given general formula of square planar complex.
Important Questions on Coordination Compounds
EASY
EASY
EASY
EASY
What type of isomerism is shown by the following coordination compounds:
and
Write their IUPAC names.
HARD
HARD
MEDIUM
HARD
(en )
EASY
MEDIUM
MEDIUM
EASY
MEDIUM
Three arrangements are shown for the complex, Which one is the wrong statement-
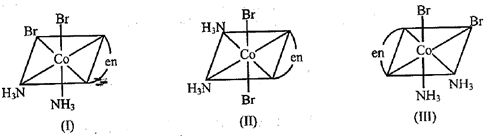
EASY
MEDIUM
EASY
MEDIUM
Both geometrical and optical isomerisms are shown by
MEDIUM
will form how many geometrical isomers?
MEDIUM
HARD

