Calculate the relative mass of .

Important Questions on Cambridge IGCSE Exam Questions from Paper 2
Equal sized pieces of magnesium, strontium and calcium are placed in water. Some observations about these reactions are shown in the table. Complete the box for strontium.
| Metal | Observations |
| Magnesium | Gives off a bubbles of gas with hot water. Dissolves very slowly. |
| Calcium | Gives of bubbles steadily with cold water. Dissolves slowly. |
| Strontium |
Complete and balance the equation for the reaction of calcium hydroxide with hydrochloric acid.
What type of chemical reaction is this?
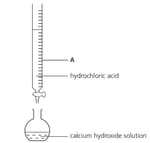
A student used the apparatus shown below to calculate the concentration of a solution of calcium hydroxide. Name the piece of apparatus labelled A.
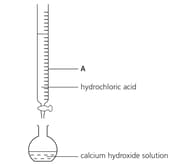
A student used the apparatus shown below to calculate the concentration of a solution of calcium hydroxide. Describe how the pH of the solution in the flask changes as the hydrochloric acid is added.
Petroleum is a mixture of hydrocarbons. It is separated into fractions such as petrol, paraffin and diesel. Name the process used to separate the fractions.
