EASY
CUET (UG)
IMPORTANT
Earn 100
Calculate the shortest wavelength of Lyman series. Given Rydberg constant, .
(a)
(b)
(c)
(d)
100% studentsanswered this correctly

Important Questions on Atoms
EASY
CUET (UG)
IMPORTANT
MEDIUM
CUET (UG)
IMPORTANT
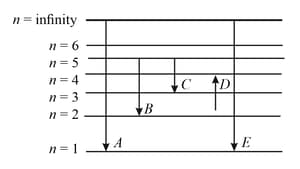
The energy levels of the hydrogen spectrum is shown in the figure. There are some transitions ,, , and . Transition , and respectively represent
EASY
CUET (UG)
IMPORTANT
MEDIUM
CUET (UG)
IMPORTANT
MEDIUM
CUET (UG)
IMPORTANT
EASY
CUET (UG)
IMPORTANT
EASY
CUET (UG)
IMPORTANT
EASY
CUET (UG)
IMPORTANT
