MEDIUM
JEE Main/Advance
IMPORTANT
Earn 100
Calculate the total entropy change for the transition at of of sulphur from the monoclinic to the rhombic solid state and for the transition. Assume the surroundings to be an ice-water bath at
(a)
(b)
(c)
(d)None of these
50% studentsanswered this correctly

Important Questions on Thermodynamics
MEDIUM
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
If for
and for
What is the Gibbs free energy of formation of
MEDIUM
JEE Main/Advance
IMPORTANT
Given the following data:
| Substance | ||
| (Graphite) | ||
Determine at what temperature the following reaction is spontaneous?
EASY
JEE Main/Advance
IMPORTANT
The following curve represents the variation of Gibbs function '' with pressure at constant temperature.
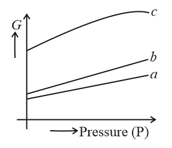
Correct match of given plots with the physical state of a substance is
EASY
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
