HARD
Earn 100
Calculate the work done during combustion of 0.138 kg of ethanol, at 300 K. (Given: , molar mass of ethanol .)
(a)– 7482 J
(b)7482 J
(c)– 2494 J
(d)2494 J
50% studentsanswered this correctly
Important Questions on Construct and interpret graphical displays of data to describe the relationships of kinetic energy to the mass of an object and to the speed of an object.
EASY
Physical Sciences>Energy>Construct and interpret graphical displays of data to describe the relationships of kinetic energy to the mass of an object and to the speed of an object.>Definitions of Energy - Motion energy is properly called kinetic energy; it is proportional to the mass of the moving object andgrows with the square of its speed.
EASY
Physical Sciences>Energy>Construct and interpret graphical displays of data to describe the relationships of kinetic energy to the mass of an object and to the speed of an object.>Definitions of Energy - Motion energy is properly called kinetic energy; it is proportional to the mass of the moving object andgrows with the square of its speed.
(i) A: in which N identical bulbs are connected in series across a battery of emf E.
(ii) B: in which N bulbs identical to those in A are connected in parallel across similar battery of emf E.
: Power dissipating in each bulb in A.
: Power dissipating in each bulb in B.
: Total power delivered by battery in circuit A.
: Total power delivered by battery in circuit B.
EASY
Physical Sciences>Energy>Construct and interpret graphical displays of data to describe the relationships of kinetic energy to the mass of an object and to the speed of an object.>Definitions of Energy - Motion energy is properly called kinetic energy; it is proportional to the mass of the moving object andgrows with the square of its speed.
MEDIUM
Physical Sciences>Energy>Construct and interpret graphical displays of data to describe the relationships of kinetic energy to the mass of an object and to the speed of an object.>Definitions of Energy - Motion energy is properly called kinetic energy; it is proportional to the mass of the moving object andgrows with the square of its speed.
EASY
Physical Sciences>Energy>Construct and interpret graphical displays of data to describe the relationships of kinetic energy to the mass of an object and to the speed of an object.>Definitions of Energy - Motion energy is properly called kinetic energy; it is proportional to the mass of the moving object andgrows with the square of its speed.
EASY
Physical Sciences>Energy>Construct and interpret graphical displays of data to describe the relationships of kinetic energy to the mass of an object and to the speed of an object.>Definitions of Energy - Motion energy is properly called kinetic energy; it is proportional to the mass of the moving object andgrows with the square of its speed.
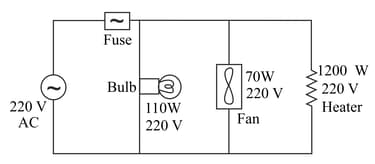
EASY
Physical Sciences>Energy>Construct and interpret graphical displays of data to describe the relationships of kinetic energy to the mass of an object and to the speed of an object.>Definitions of Energy - Motion energy is properly called kinetic energy; it is proportional to the mass of the moving object andgrows with the square of its speed.
EASY
Physical Sciences>Energy>Construct and interpret graphical displays of data to describe the relationships of kinetic energy to the mass of an object and to the speed of an object.>Definitions of Energy - Motion energy is properly called kinetic energy; it is proportional to the mass of the moving object andgrows with the square of its speed.
EASY
Physical Sciences>Energy>Construct and interpret graphical displays of data to describe the relationships of kinetic energy to the mass of an object and to the speed of an object.>Definitions of Energy - Motion energy is properly called kinetic energy; it is proportional to the mass of the moving object andgrows with the square of its speed.
HARD
Physical Sciences>Energy>Construct and interpret graphical displays of data to describe the relationships of kinetic energy to the mass of an object and to the speed of an object.>Definitions of Energy - Motion energy is properly called kinetic energy; it is proportional to the mass of the moving object andgrows with the square of its speed.
If the current through a resistor is increased by 50%, the increase in power dissipated will be (assume the temperature remains constant)
EASY
Physical Sciences>Energy>Construct and interpret graphical displays of data to describe the relationships of kinetic energy to the mass of an object and to the speed of an object.>Definitions of Energy - Motion energy is properly called kinetic energy; it is proportional to the mass of the moving object andgrows with the square of its speed.
EASY
Physical Sciences>Energy>Construct and interpret graphical displays of data to describe the relationships of kinetic energy to the mass of an object and to the speed of an object.>Definitions of Energy - Motion energy is properly called kinetic energy; it is proportional to the mass of the moving object andgrows with the square of its speed.
HARD
Physical Sciences>Energy>Construct and interpret graphical displays of data to describe the relationships of kinetic energy to the mass of an object and to the speed of an object.>Definitions of Energy - Motion energy is properly called kinetic energy; it is proportional to the mass of the moving object andgrows with the square of its speed.
A water pump lifts water from a level below the ground. The water is pumped at the rate of with negligible velocity. Calculate the minimum power the pump should have to do this work.
EASY
Physical Sciences>Energy>Construct and interpret graphical displays of data to describe the relationships of kinetic energy to the mass of an object and to the speed of an object.>Definitions of Energy - Motion energy is properly called kinetic energy; it is proportional to the mass of the moving object andgrows with the square of its speed.
EASY
Physical Sciences>Energy>Construct and interpret graphical displays of data to describe the relationships of kinetic energy to the mass of an object and to the speed of an object.>Definitions of Energy - Motion energy is properly called kinetic energy; it is proportional to the mass of the moving object andgrows with the square of its speed.
EASY
Physical Sciences>Energy>Construct and interpret graphical displays of data to describe the relationships of kinetic energy to the mass of an object and to the speed of an object.>Definitions of Energy - Motion energy is properly called kinetic energy; it is proportional to the mass of the moving object andgrows with the square of its speed.
MEDIUM
Physical Sciences>Energy>Construct and interpret graphical displays of data to describe the relationships of kinetic energy to the mass of an object and to the speed of an object.>Definitions of Energy - Motion energy is properly called kinetic energy; it is proportional to the mass of the moving object andgrows with the square of its speed.
EASY
Physical Sciences>Energy>Construct and interpret graphical displays of data to describe the relationships of kinetic energy to the mass of an object and to the speed of an object.>Definitions of Energy - Motion energy is properly called kinetic energy; it is proportional to the mass of the moving object andgrows with the square of its speed.
EASY
Physical Sciences>Energy>Construct and interpret graphical displays of data to describe the relationships of kinetic energy to the mass of an object and to the speed of an object.>Definitions of Energy - Motion energy is properly called kinetic energy; it is proportional to the mass of the moving object andgrows with the square of its speed.
HARD
Physical Sciences>Energy>Construct and interpret graphical displays of data to describe the relationships of kinetic energy to the mass of an object and to the speed of an object.>Definitions of Energy - Motion energy is properly called kinetic energy; it is proportional to the mass of the moving object andgrows with the square of its speed.

