HARD
Earn 100
Camphor in nitrogen gas is an example of solid in gas solutions.
(a)True
(b)False
87.5% studentsanswered this correctly
Important Questions on Solutions
EASY
HARD
EASY
MEDIUM
Radha prepared a solution which could be separated by filtration.
(i) Name the type of solution
(ii) Is the solution transparent or opaque?
(iii) Mention the nature of the solution.
(iv Mention the size of the solute particles.
EASY
MEDIUM
Consider the following statements about Tincture of Iodine:
It is an antiseptic solution.
Iodine is kept in alcohol-water mixture.
Concentration of iodine is very low.
How many of the above statements is/are correct?
MEDIUM
EASY
MEDIUM
EASY
MEDIUM
MEDIUM
EASY
MEDIUM
Match the items given in Column I and Column II.
| Column I | Column II |
| (i) Saturated solution | (a) Solution having same osmotic pressure at a given temperature as that of given solution. |
| (ii) Binary solution | (b) A solution whose osmotic pressure is less than that of another. |
| (iii) Isotonic solution | (c) Solution with two components. |
| (iv) Hypotonic solution | (d) A solution which contains maximum amount of solute that can be dissolved in a given amount of solvent at a given temperature. |
| (v) Solid solution | (e) A solution whose osmotic pressure is more than that of another. |
| (vi) Hypertonic solution | (f) A solution in solid phase. |
MEDIUM
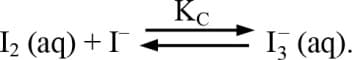 .
.What is the value of ?
EASY

