EASY
10th CBSE
IMPORTANT
Earn 100
Can rusting of iron take place in distilled water?

Important Questions on Chemical Reactions And Equations
EASY
10th CBSE
IMPORTANT
The value of in the balance chemical equation is:
EASY
10th CBSE
IMPORTANT
EASY
10th CBSE
IMPORTANT
MEDIUM
10th CBSE
IMPORTANT
Among the following, the number of underlined elements having oxidation state are:
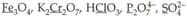
MEDIUM
10th CBSE
IMPORTANT
EASY
10th CBSE
IMPORTANT
Nandita mixed two solutions and . She recorded the following observations and conclusions in her notebook.
A yellow precipitate is formed.
It is a double displacement reaction.
The solutions and respectively are:
EASY
10th CBSE
IMPORTANT
Which of the following statements is wrong relating to the above equation?
EASY
10th CBSE
IMPORTANT
Which of the following are exothermic processes?
Reaction of water with quick lime.
Dilution of an acid.
Evaporation of water.
Sublimation of camphor.
