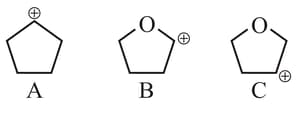
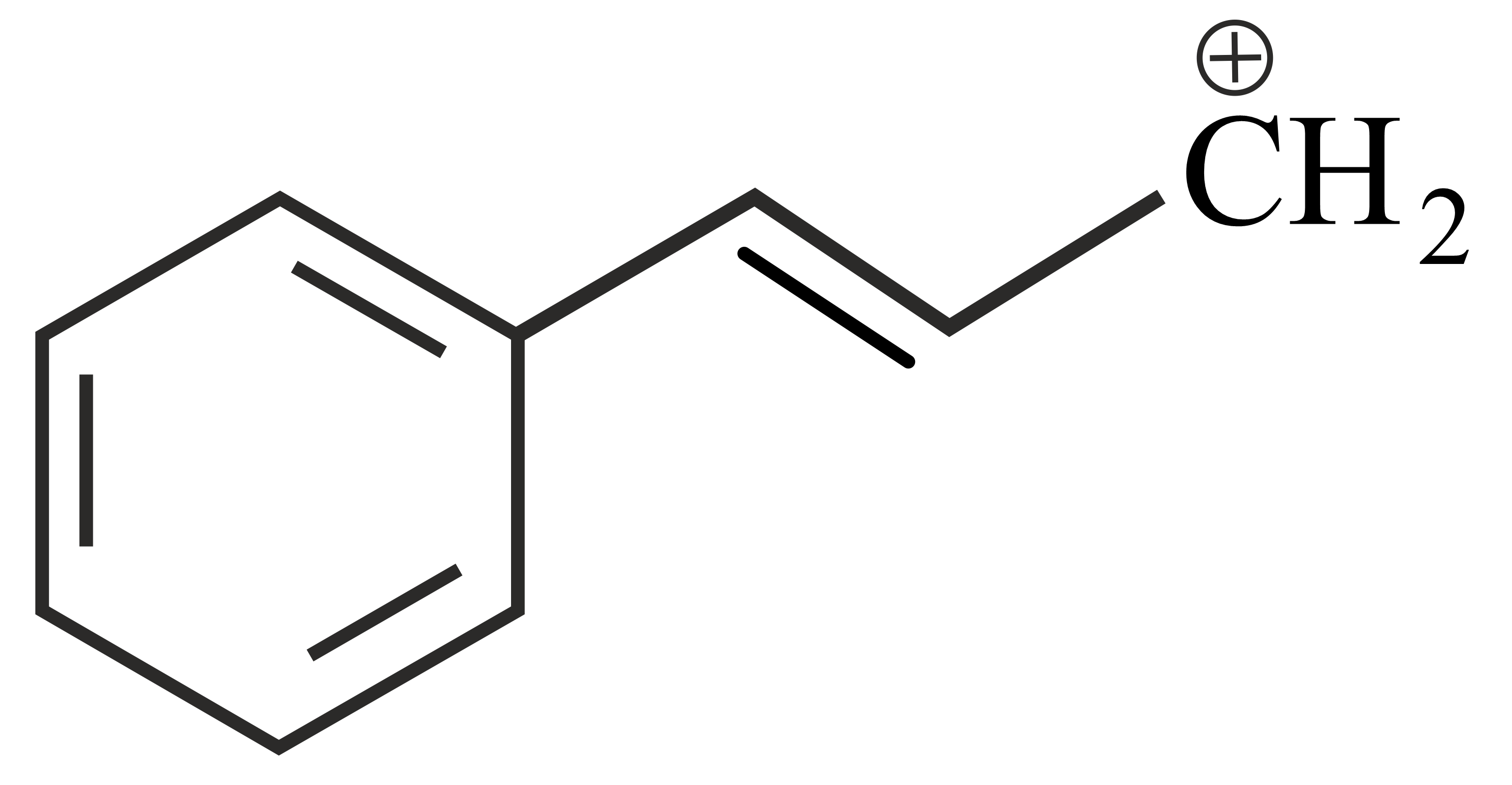
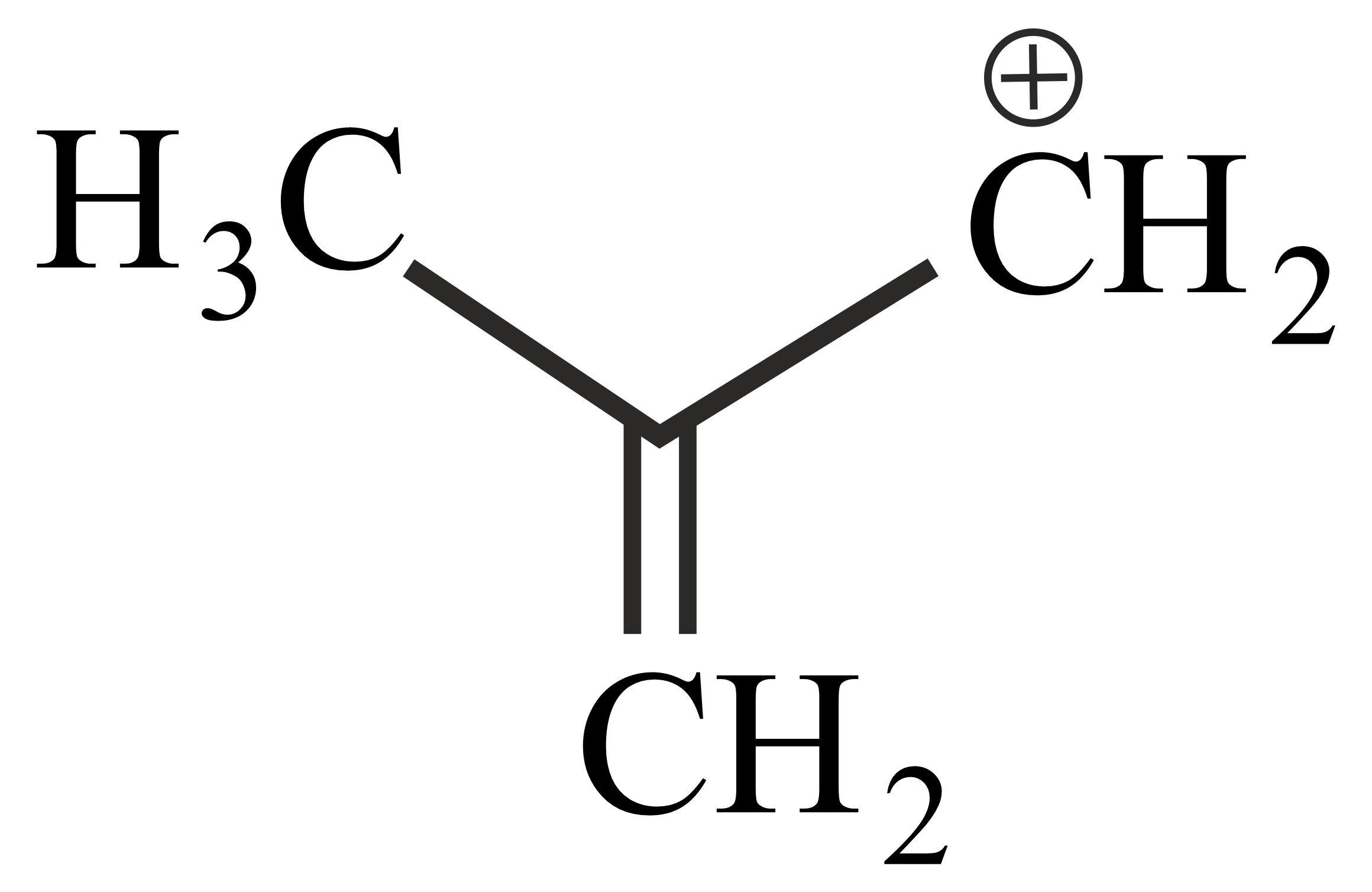
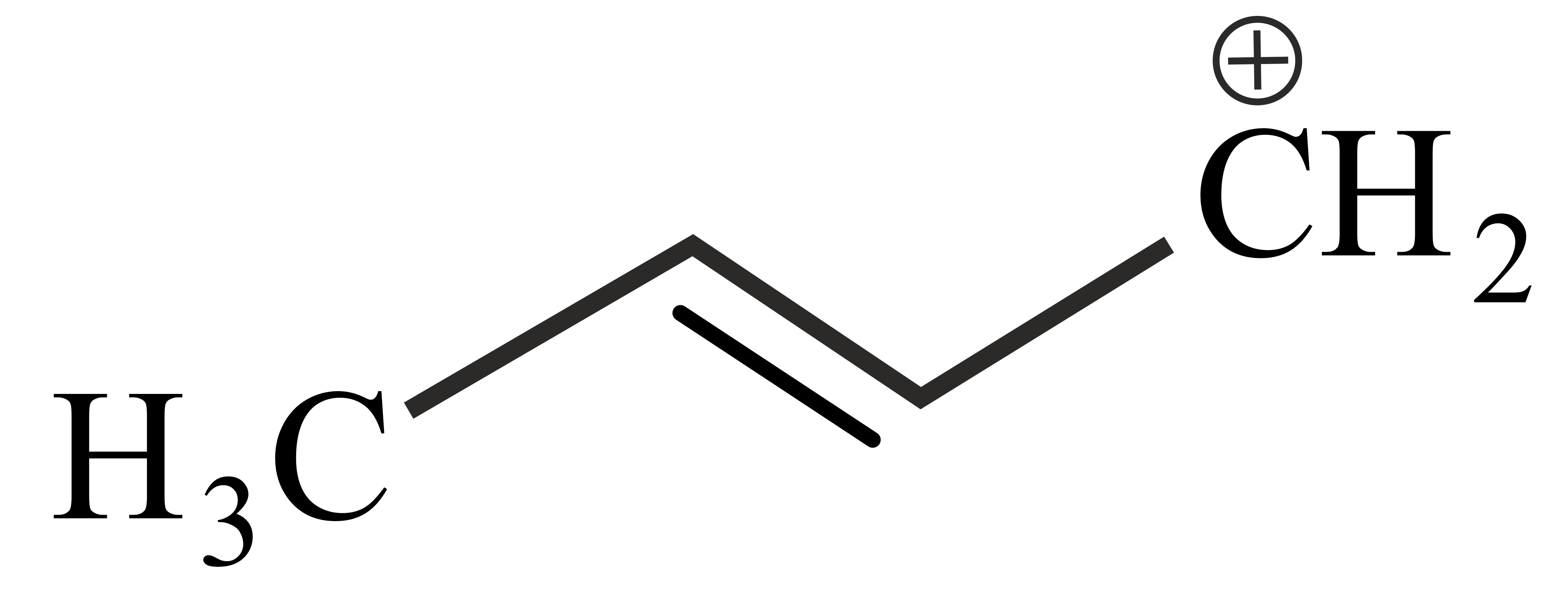
Carbocations are important intermediates in organic reactions, they are often stabilised by resonance, inductive effect and hyperconjugation. The cation that can be stabilised by hyperconjugation is
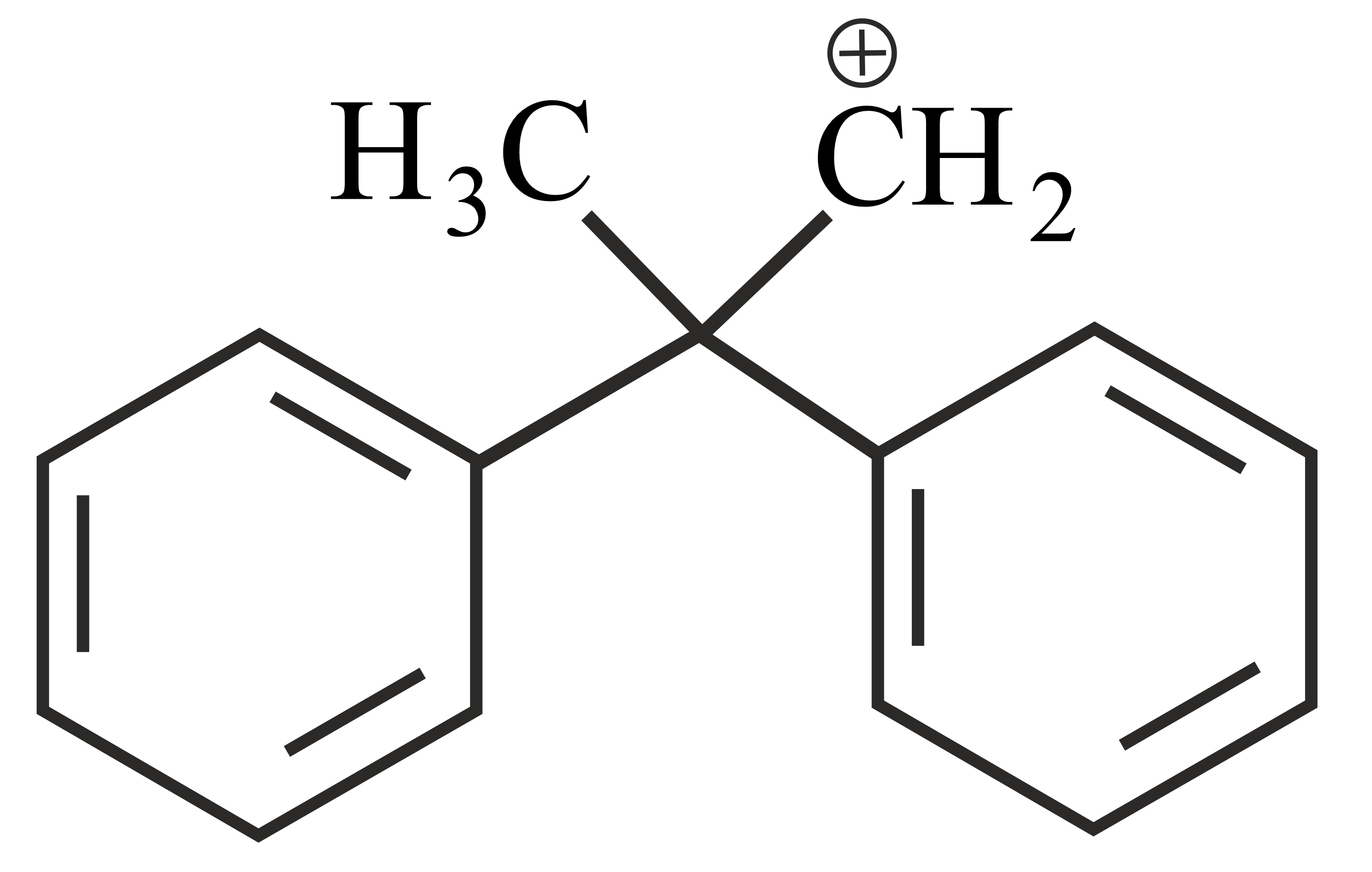
Important Questions on Organic Chemistry- Some Basic Principles and Techniques
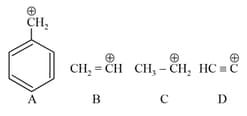
The correct order of stability of given carbocation is:
Which of the following carbocations is most stable?
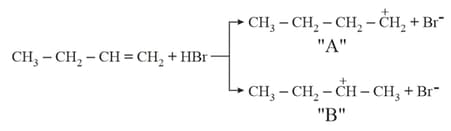
Choose the correct statement regarding the formation of carbocations A and B given :-


follows the order
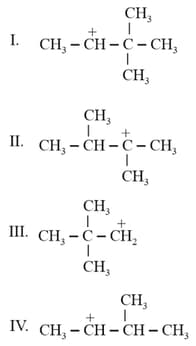
The decreasing order of the stability of the following carbocations is
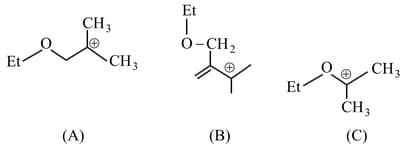
The stability order of the above carbocations is
Which of the following is most stable
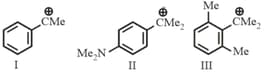
I.
II.
III.
Assertion (A): Tertiary carbocations are more reactive than secondary and primary carbocations.
Reason (R): Hyper conjugation, as well as inductive effect due to additional alkyl groups stabilise tertiary carbocations.
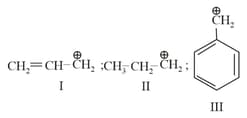
Consider the following carbocations
(a)
(b)
(c)
(d)
The stability sequence follows the order
Carbanions derived from aldehydes/ketones are called enolates, as they represent anions (conjugate bases) of the enol forms. Enol ethers can be prepared and are synthetically important. These anions react with an alkyl halide at the carbon or oxygen atom.
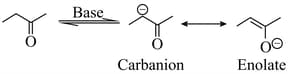
Trimethylsilylchloride reacts almost exclusively with enolates. One such reaction is shown below. where ketone is treated with LDA. followed by to obtain trimethylsilyl derivative of . The possible intermediate carbanions (and ) and the possible products ( to ) are shown. [LDA lithium diisopropylamide , is a strong base but a very poor nucleophile].
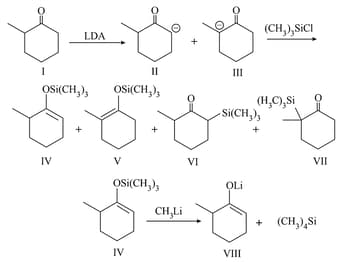
Arrange the following carbocations in decreasing order of stability.