HARD
Earn 100
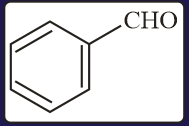
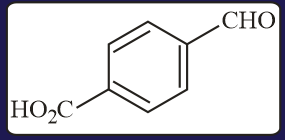
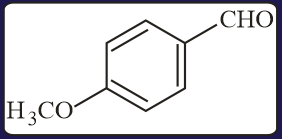
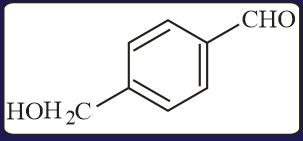
Carbonyl compounds, sensitive to both acids and bases, can be reduced to hydrocarbons by
(a)Clemmensen reduction
(b)Wolf-Kishner reduction
(c)Thioacetal reduction
(d)All of the three
50% studentsanswered this correctly
Important Questions on Hydrocarbons
HARD
EASY
MEDIUM
MEDIUM
EASY
EASY
Which of the following reactions is used for producing symmetrical alkane with double carbon atoms?
HARD
Isomers of hexane, based on their branching, can be divided into three distinct classes as shown in the figure.
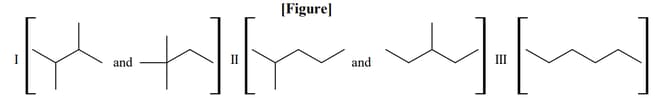
The correct order of their boiling point is
EASY
EASY
MEDIUM
Which hydrogen in compound is easily replaceable during bromination reaction in presence of light ?
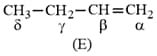
MEDIUM
EASY
MEDIUM
EASY
MEDIUM
MEDIUM
MEDIUM
The aldehydes which will not form Grignard product with one equivalent of Grignard reagents are