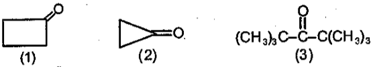MEDIUM
NEET
IMPORTANT
Earn 100
Carbonyl compounds undergo nucleophilic addition because of
(a)electronegativity difference of carbon and oxygen atoms
(b)electromeric effect
(c)more stable anion with negative charge on oxygen atom and less stable carbonium ion
(d) All
50% studentsanswered this correctly

Important Questions on Aldehydes, Ketones and Carboxylic Acids
MEDIUM
NEET
IMPORTANT
Acetoactic ester will give iodoform test
It contains group
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
: Benzaldehyde is more reactive than ethanol towards nucleophilic attack.
The overall effect of and effect of phenyl group decreases the electron density on the carbon atom of group in benzaldehyde.
MEDIUM
NEET
IMPORTANT
In the following reaction
Identify and .
MEDIUM
NEET
IMPORTANT
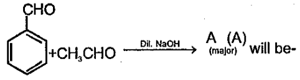
Maior product is
MEDIUM
NEET
IMPORTANT
Benzaldehyde is less reactive in comparison to ethanol towards nucleophilic attack.
All the carbon atoms of benzaldehyde are hybridised.
MEDIUM
NEET
IMPORTANT
Benzaldehyde is more reactive than ethanol towards nuclephilic attack.
The overall effect of and effect of phenyl group decreases the electron density on the carbon atom of 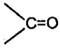 .
.
MEDIUM
NEET
IMPORTANT
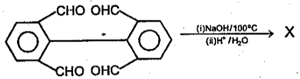
Arrange the following in the order of increasing value of the equilibrium constant for hydration (smallest value first):