HARD
Earn 100
Carnot cycle works with isentropic compression ratio of and heat capacity ratio of . The volume of air at the beginning of the isothermal expansion is. If the temperature and pressure is limited to and , determine minimum temperature in the cycle.
Important Questions on Thermodynamics
MEDIUM
MEDIUM
Helium gas goes through a cycle (consisting of two isochoric and two isobaric lines) as shown in figure. The efficiency of this cycle is approximately
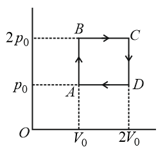
EASY
MEDIUM
MEDIUM
EASY
EASY
MEDIUM
HARD
EASY
HARD
MEDIUM
EASY
For a Carnot engine which of the following is false?
EASY
MEDIUM
A Carnot engine is operating between a hot body and cold body maintained at temperature and respectively. Consider the following three cases
Case I : The temperature of the hot body is changed to and cold body is at
Case II : The temperature of the hot body is at and cold body is changed to
Case III : The temperature of the hot body is
EASY
HARD
MEDIUM
EASY
EASY

