Chlorine gas was prepared using electrolysis of brine solution. Write the chemical equation to represent the change. Identify the other products formed in the process and give one application for each.

Important Questions on Acids, Bases and Salts
A student was given three solutions marked X, Y and Z, and asked to arrange them in the increasing order of their pH values. The student put two drops of each solution on three strips of universal indicator paper separately. The colours shown by the three indicator strips are as follows:
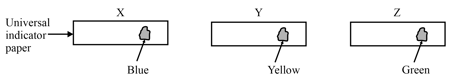
Which of the following gives the correct order of increasing pH values.
Which of the following statements is correct about an aqueous solution of an acid and of a base?
(i) Higher the pH, stronger the add (ii) Higher the pH, weaker the acid
(iii) Lower the pH, stronger the base (iv) Lower the pH, weaker the base
In an experiment to measure the pH values of solutions, a student placed strips of universal indicator paper in four beakers containing solutions A, B, C and D. The colour of universal indicator paper in these solutions is as shown below:
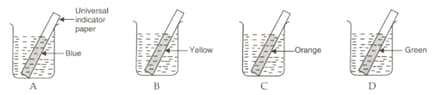
The solution having lowest pH value is :
