Chlorofluorocarbons (or CFCs) are chemicals that destroy the ozone in the atmosphere. A common CFC has a chemical formula . Using the table below, what is the relative formula mass of ?
Chemical symbol
Element
Relative atomic mass
Carbon
Chlorine
Fluorine
Important Questions on Atoms and Molecules
type of semi-conductor material
amount of doping
temperature
Which one of the following is correct?
Ethane burns in oxygen to form and according to the equation . If of oxygen is burnt with of ethane. Calculate the volume of unused .
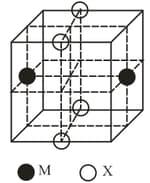
The cubic unit cell structure of a compound containing cation and anion is shown below. When compared to the anion, the cation has smaller ionic radius. Choose the correct statement(s).
Aluminium carbide reacts with water according to the following equation:
What volume of methane at s.t.p. is obtained from of aluminium carbide? [Relative molecular weight of ]
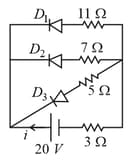
In the following circuit diagram, the current through the battery is
Match the following
List - I List-II
A) Metallic solid I) Carbon tetrachloride
B) Covalent solid II) Calcium fluoride
C) Molecular solid III) Copper
D) Ionic solid IV) Silicon carbide
V) Glass
The correct answer is
Remove all the anions , except the central one.
Replace all the face centered cations , by anions .
Remove all the corner cations .
Replace the central anion , with cation .
The value of in is _____.
Aluminium carbide reacts with water according to the following equation: .
What mass of aluminium hydroxide is formed from of aluminium carbide?
Nitroglycerine is used as an explosive. The equation for the explosive reaction is:
(Atomic mass of C = 12, H = 1, N = 14, O = 16)
What is the mass of 1 mole of glycerine?

