HARD
Earn 100
Choose the correct options:
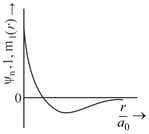
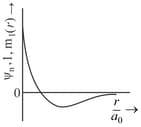
(a)Sum of radial and angular nodes in orbital is .
(b)Number of electrons having magnetic quantum number equal to zero are in .
(c)Orbital angular momentum of and orbitals is the same.
(d)Orbital angular momentum in shell is .
50% studentsanswered this correctly
Important Questions on Structure of Atom
EASY
EASY
MEDIUM
MEDIUM
EASY
MEDIUM
MEDIUM
MEDIUM
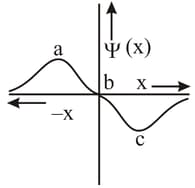
EASY
MEDIUM
How many of the following orbitals have two angular and one radial nodes?
MEDIUM
HARD
| Column 1 | Column 2 | Column 3 |
| (I) orbital | (i) |
(P) |
| (II) orbital | (ii) One radial node | (Q) Probability density at the nucleus . |
| (III) orbital | (iii) | (R) Probability density is maximum at the nucleus. |
| (IV) orbital | (iv) -plane is a nodal plane | (S) Energy needed to excite an electron from state to state is times the energy needed to excite are electron from state to state. |
MEDIUM
EASY
HARD
| Column – 1 | Column – 2 | Column – 3 |
| (I) orbital | (i) |
(P) |
| (II) orbital | (ii) One radial node | (Q) Probability density at nucleus |
| (III) orbital | (iii) | (R) Probability density is maximum at nucleus |
| (IV) orbital | (iv) -plane is a nodal plane | (S) Energy needed to excite an electron from state to state is times the energy needed to excite electron from state to state. |
EASY
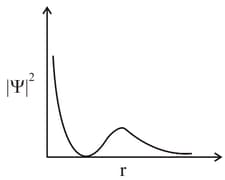
MEDIUM
EASY
HARD
EASY