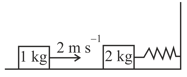
Choose the correct statement(s):
Important Questions on Kinetic Theory of Gases and Radiation
Define the following term : Brownian movement.
Consider an ideal gas confined in an isolated closed chamber. As the gas undergoes an adiabatic expansion, the average time of collision between molecules increases as , where is the volume of the gas. The value of is:
Name the following phenomenon/process :
Zig-zag movement of colloidal particles
( is universal gas constant and is the acceleration due to gravity)
Some smoke is trapped in a small glass container and is viewed through a microscope. A number of very small smoke particles are seen in continuous random motion as a result of their bombardment by air molecules. If the mass of the smoke particle is about times higher than that of an air molecule the average speed of a smoke particle is
Which of the following curves represent the variation of coefficient of volume expansion of an ideal gas at constant pressure?