EASY
Earn 100
Choose the correct statement for processes & shown in figure.
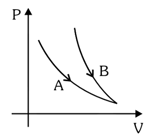

(a) for process and for process .
(b) for process and .
(c) for process and for process .
(d) for process and for process .
68.22% studentsanswered this correctly
Important Questions on Thermodynamics
MEDIUM
MEDIUM
EASY
EASY
For the given cyclic process as shown for a gas, the work done is:
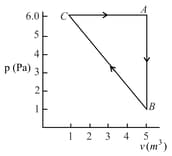
EASY
MEDIUM
EASY
EASY
One mole of an ideal diatomic gas undergoes a transition from to along a path as shown in the figure,
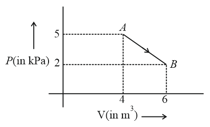
The change in internal energy of the gas during the transition is:
EASY
HARD
EASY
EASY
EASY
(Take gas constant )
MEDIUM
MEDIUM
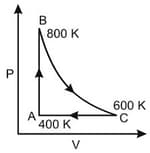
One mole of diatomic ideal gas undergoes a cyclic process ABC as shown in figure. The process BC is adiabatic. The temperatures at A, B and C are 400 K, 800 K and 600 K respectively. Choose the correct statement :
HARD
MEDIUM
An ideal gas is taken reversibly around the cycle as shown on the T (temperature) - S (entropy) diagram
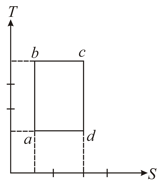
The most appropriate representation of above cycle on a U (internal energy)-V (volume) diagram is
EASY
EASY
MEDIUM

