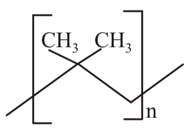
MEDIUM
Earn 100
Choose the incorrect statements about the following alkenes is/are
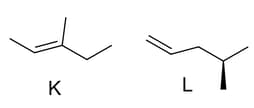

(a)Alkene K is more stable than L
(b)Hydrogenation of achiral alkene K gives chiral product.
(c)Hydrogenation of achiral alkene L gives chiral product.
(d)The two alkenes K and L are structural isomers
50% studentsanswered this correctly
Important Questions on Hydrocarbons
EASY
MEDIUM
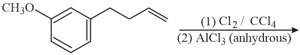
The major products obtained in the following reaction is/are
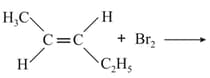
EASY
EASY
Name the gas evolved in the following case:
Ethene undergoes hydrogenation reaction.
MEDIUM
HARD
EASY
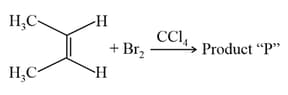
Consider the above chemical reaction. The total number of stereoisomers possible for Product "P" is _________
MEDIUM

EASY
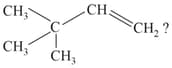
EASY
Write a balanced chemical equation the following reaction:
Chlorine gas is reacted with ethene.
HARD
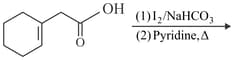
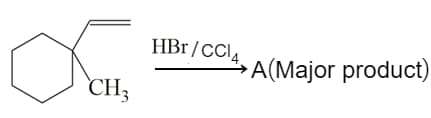
Find out the major product for the above reaction.
HARD
Amongst the following, the major product of the given chemical reaction is
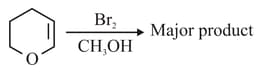
MEDIUM
The correct statement(s) for the following addition reactions is(are)
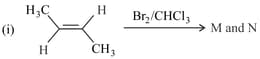
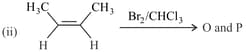
EASY
MEDIUM
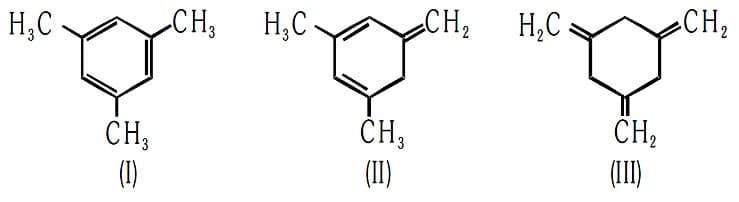
The enthalpy of hydrogenation of these compounds will be in the order as: