MEDIUM
Earn 100
has completely filled K and L shells. Explain.
Important Questions on Structure of The Atom
EASY
MEDIUM
The schematic atomic structure of four elements is given below. Observe and choose the right statement.
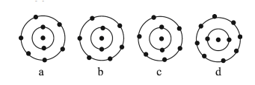
MEDIUM
MEDIUM
Match the following :
List -I :
(a) Frequency of distribution of the emitted radiation from a black body
(b) Spin quantum numbers(ms)
(c) Angular Momentum
(d) All orbital have equal energy
List - II :
(i) degeneracy
(ii) temperature dependent
(iii) vector quantity
(iv) mass times velocity times radius
Codes:
MEDIUM
EASY
MEDIUM
An isoelectronic species are
MEDIUM
MEDIUM
MEDIUM
MEDIUM
Two atoms are said to be isobars if
EASY
MEDIUM
EASY
MEDIUM
MEDIUM
EASY
MEDIUM
MEDIUM
HARD
Nitrogen (atomic number ) and phosphorus (atomic number ) belong to group of the Periodic Table. Write the electronic configuration of these two elements. Which one of these will be more electronegative? Why?
