EASY
Earn 100
Co-ordination number of atoms in is:
(a)
(b)
(c)
(d)
50% studentsanswered this correctly
Important Questions on Solid State
MEDIUM
MEDIUM
The edge length of a solid possessing cubic unit cell is (structure I), based on hard sphere model, which upon subjecting to a phase transition, a new cubic structure (structure II) having an edge length of is obtained, where is the radius of the hard sphere. Which of the following statements is true ?
HARD
MEDIUM
MEDIUM
MEDIUM
EASY
MEDIUM
EASY

EASY
HARD
EASY
HARD
In a face centered cubic (fcc) lattice, the position which represents an octahedral hole is
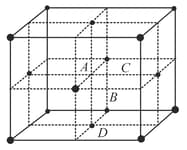
HARD
HARD
A two dimensional solid is made by alternating circles with radius and such that the sides of the circles touch. The packing fraction is defined as the ratio of the area under the circles to the area under the rectangle with sides of length and
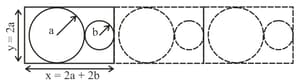
The ratio for which the packing fraction is minimized is closest to:
EASY
HARD
Hexagonal close-packing and cubic close-packing?
MEDIUM
EASY
MEDIUM

