MEDIUM
Earn 100
Collect and paste the available pictures of matter
Important Questions on Matter is Everywhere
EASY
HARD
EASY
MEDIUM
MEDIUM
EASY
type of semi-conductor material
amount of doping
temperature
Which one of the following is correct?
HARD
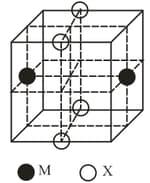
Remove all the anions , except the central one.
Replace all the face centered cations , by anions .
Remove all the corner cations .
Replace the central anion , with cation .
The value of in is _____.
EASY
In the following circuit diagram, the current through the battery is
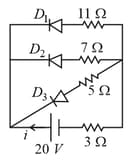
MEDIUM
Based on the given figure, the number of correct statement/s is/are
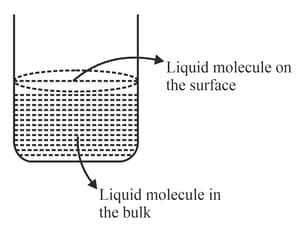
A. Surface tension is the outcome of equal attractive and repulsion forces acting on the liquid molecule in bulk.
B. Surface tension is due to uneven forces acting on the molecules present on the surface.
C. The molecule in the bulk can never come to the liquid surface.
D. The molecules on the surface are responsible for vapour pressure if the system is a closed system.
EASY
HARD
The cubic unit cell structure of a compound containing cation and anion is shown below. When compared to the anion, the cation has smaller ionic radius. Choose the correct statement(s).
HARD
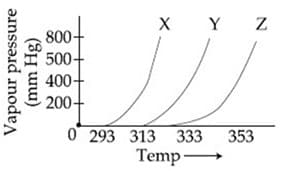
The following inferences are made:
has higher intermolecular interactions compared to
has lower intermolecular interactions compared to
has lower intermolecular interactions compared to
The correct inferences is/are:
EASY
MEDIUM
EASY
EASY
MEDIUM
MEDIUM
MEDIUM

