EASY
MHT-CET
IMPORTANT
Earn 100
Collision theory is applicable to _____ reactions.
(a)first order
(b)zero order
(c)bimolecular
(d)intra molecular
68.75% studentsanswered this correctly

Important Questions on Chemical Kinetics
EASY
MHT-CET
IMPORTANT
If is the fraction of molecules having energy greater than , the relation between the two will be given by _____.
EASY
MHT-CET
IMPORTANT
Energy of activation of an exothermic reaction is _____.
EASY
MHT-CET
IMPORTANT
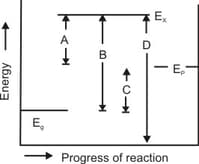
In the accompanied diagram, represent the energy of the reactants, products and activated complex, respectively. Which of the following is the activation energy for the backward reaction?
EASY
MHT-CET
IMPORTANT
The rate constant of one of the reaction is found to be double that of the rate constant of another reaction. Then, the relationship between the corresponding activation energies of the two reactions ( and ) can be represented as _____.
EASY
MHT-CET
IMPORTANT
What happens when the temperature of a solution is increased from ?
EASY
MHT-CET
IMPORTANT
The rate constant is given by the equation . Which factor should register a decrease for the reaction to proceed more rapidly?
MEDIUM
MHT-CET
IMPORTANT
The rate constants at for the dissociation of respectively. What is the activation energy for the dissociation of ?
EASY
MHT-CET
IMPORTANT
Which of the following is NOT the function of catalyst?
