MEDIUM
Earn 100
Colourless salt . (X) can be
(a)Borax
(b)Microcosmic salt
(c)Both Borax and Microcosmic salt
(d)None of these
50% studentsanswered this correctly
Important Questions on Theoretical Principles of Experimental Chemistry
MEDIUM
The green colour produced in the borax bead test of a chromium(III) salt is due to -
EASY
Match List-I with List-II and select the correct answer using the codes given below.
| List I | List II | ||
| A. | Rinmann's green | 1. | |
| B. | Verdigris | 2. | |
| C. | Corrosive sublimate | 3. | |
| D. | Lithopone | 4. |
MEDIUM
State one relevant observation for of the following:
Anhydrous calcium chloride is exposed to air for some time.
EASY
The hottest region of Bunsen flame shown in the figure below is:
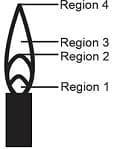
MEDIUM
Which of the following salt gives white precipitate with solution and dil. solution and gives green flame test
EASY
When the salt is heated in a dry test tube sublimation yields a solid substance in the upper, colder parts of the test tube. The deposit is yellow in colour. Therefore salt contain
MEDIUM
When a substance 'A' reacts with water, it produces a combustible gas 'B' and a solution of substance 'C' in water. While another substance 'D' reacts with solution of 'C' to produce the same gas B on warming while 'D' can produce gas 'B' on reaction with dilute at room temperature. 'A' imparts a deep golden - yellow colour to a smokeless flame on Bunsen burner. Identify 'A', 'B', 'C' and 'D' respectively are
MEDIUM
A pale yellow precipitate and a gas with pungent odour are formed on warming dilute hydrochloric acid with an aqueous solution containing :
EASY
The solubilities of carbonates decrease down the magnesium group due to a decrease in
EASY
A salt gives bright red colour to the flame. This colour indicates the presence of
EASY
Which one of the following salt give green coloured flame when the salt is tested by wire
MEDIUM
Which of the following substance is obtained as precipitate when solution is added to aqueous solution?
MEDIUM
On mixing two colourless gases, a deep brown colour is observed. The gases are
MEDIUM
The dark red colour of bombs in fireworks is due to the presence of
EASY
Which one of the following anions is not easily removed from aqueous solutions by precipitation?
EASY
A colourless gas with the smell of rotten fish is
MEDIUM
Which of the following sulphate is insoluble in water
HARD
Which of the following precipitate does not dissolve even in large excess of dilute
MEDIUM
Nitrates of all the metals are
HARD
The metal that does not give the borax bead test is

