HARD
JEE Main
IMPORTANT
Earn 100
Complete combustion of of an oxygen containing compound gave of and of The percentage of oxygen in the organic compound is :
(a)
(b)
(c)
(d)
76.19% studentsanswered this correctly

Important Questions on Organic Chemistry- Some Basic Principles and Techniques
MEDIUM
JEE Main
IMPORTANT
Using the provided information in the following paper chromatogram:
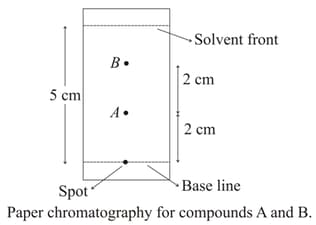
The calculated value of .
MEDIUM
JEE Main
IMPORTANT
The formula of a gaseous hydrocarbon which requires 6 times of its own volume of for complete oxidation and produces times its own volume of is The value of is ________.
MEDIUM
JEE Main
IMPORTANT
Which purification technique is used for high boiling organic liquid compound (decomposes near its boiling point) ?
HARD
JEE Main
IMPORTANT
Which of the following molecules does not show stereo isomerism ?
MEDIUM
JEE Main
IMPORTANT
The number of acyclic structural isomers (including geometrical isomers) for pentene are
EASY
JEE Main
IMPORTANT
Which one of the following pairs of isomers is an example of metamerism?
EASY
JEE Main
IMPORTANT
In Carius method, halogen containing organic compound is heated with fuming nitric acid in the presence of:
MEDIUM
JEE Main
IMPORTANT
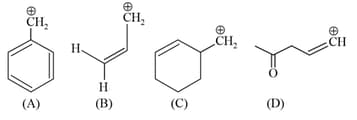
Among the given species the Resonance stabilised carbocations are:
