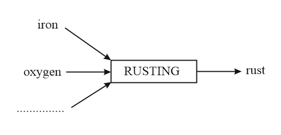
Complete the diagram shown that substances are used and what is produced in burning, respiration and rusting.


Important Questions on Chemical Reactions
A further definition links oxidation and reduction to the exchange of electrons during the reaction.
Oxidation is the _____ of electrons.
A further definition links oxidation and reduction to the exchange of electrons during the reaction.
Reduction is the _____ of electrons.
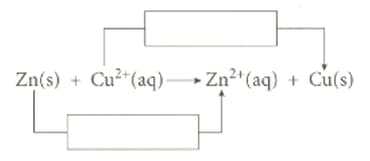
Fill the boxes on the ionic equation below with the appropriate terms.
Complete the following passage by using the words listed below:
anode, electrodes, current, molten, electrolyte, solution, cathode, positive, hydrogen, molecules, lose, oxygen.
During electrolysis, ionic compounds are decomposed by the passage of an electric current. For this to happen, the compound must be either _____ or _____ in _____. Electrolysis can occur when an electric _____ passes through a molten _____. The two rods dipping into the electrolyte are called the _____. In this situation, metals are deposited at the _____ and non-metals arc formed at the _____.
When the ionic compound is dissolved in water, the electrolysis can be more complex. Generally, during electrolysis _____ ions move towards the _____ and negative ions move towards the _____. At the negative electrode (cathode) the metal or _____ ions gain electrons and form metal atoms or hydrogen _____. At the positive electrode (anode) certain non-metal ions _____ electrons and ______ or chlorine is produced.
Complete the passage by using the words listed below:
hydrogen, hydroxide, lower, copper, sodium, molten, cryolite, purifying, positive, concentrated.
There are several important industrial applications of electrolysis, the most important economically being the electrolysis of ______ aluminium oxide to produce aluminium. The aluminium oxide is mixed with molten _____ to _____ the melting point of the electrolyte.
An ______ aqueous solution of sodium chloride contains _____ chloride, hydrogen and _____ ions. When this solution is electrolysed ______ rather than sodium is discharged at the negative electrode. The solution remaining is sodium hydroxide.
When a solution of copper(II) sulphate is electrolysed using ______ electrodes, an unusual thing happens and the copper atoms of the ______ electrode (anode) go into solution as copper ions. At the cathode, the copper ions turn into copper atoms, and the metal is deposited on this electrode. This can be used as a method of refining or _____ impure copper.
The results of displacement reactions are as follows:
(i) Rough notes: solution with bromine or iodine solutions - no change to colourless solution - hexane not added.
(ii) solution with iodine solution - no change to colourless solution -hexane not added.
(iii) solution with chlorine solution - solution colourless to brown - brown colour moves to upper layer at the end.
(iv) solution with chlorine or bromine water - solution colourless to brown in both cases - purple colour in upper hexane layer at the end ( brown colour of the aqueous layer reduced)
Take these recorded observations and draw up a table of the results. If there is no change, then write 'no reaction'.
