MEDIUM
11th Maharashtra Board
IMPORTANT
Earn 100
Complete the flow chart.
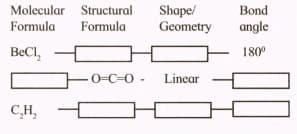


Important Questions on Chemical Bonding
HARD
11th Maharashtra Board
IMPORTANT
Complete the following table.
| Molecule | Type of Hybridisation | Type of bonds | Geometry | Bond angle |
| - | bonds | Tetrahedral | - | |
| - | - | |||
| - | - | Angular | ||
| - | - | |||
| - | - | - | ||
| - | Linear | - | ||
| - | - |
MEDIUM
11th Maharashtra Board
IMPORTANT
Indicate the factor on which stability of ionic compound is measured?
MEDIUM
11th Maharashtra Board
IMPORTANT
MEDIUM
11th Maharashtra Board
IMPORTANT
MEDIUM
11th Maharashtra Board
IMPORTANT
MEDIUM
11th Maharashtra Board
IMPORTANT
MEDIUM
11th Maharashtra Board
IMPORTANT
MEDIUM
11th Maharashtra Board
IMPORTANT
