Complete the following statements:
Phosphoric acid always produces ______.

Important Questions on Acids, Bases and Salts
Complete the table below which summarises the products of various reactions of acids.
| Substances react together | salt produced | other products of the reaction | |
| Dilute hydrochloric acid | zinc oxide | ||
| dilute sulphuric acid | copper sulphate | water and carbon dioxide | |
| magnesium sulphate | water and carbon dioxide | ||
| magnesium chloride | hydrogen | ||
| dilute nitric acid | copper oxide | ||
| dilute ethanoic acid | sodium ethanoate | water | |
| potassium hydroxide | potassium phosphate | ||
Search the Internet to find the answer for the following question:
What is the formula for sulfamic acid and what is it used for?
Search the Internet to find the answer for the following question:
Why does water sometimes produce calcium carbonate(limescale) when it is heated? What is hard water?
Insoluble salts can be made using a precipitation reaction. The method can be used to find the formula of a salt. In an experiment, of a solution of the nitrate of metal X was placed in a narrow test tube and of aqueous sodium phosphate, was added. The precipitate settled and its height was measured. The concentration of both solutions was .
The experiment was repeated using different volumes of the sodium phosphate solution. The results are shown on the graph.
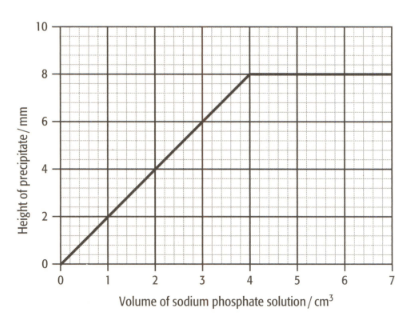
What is the formula of the phosphate of metal X? Give your reasoning.
